Improving Patient Experience, Enhancing Data-Driven Insights, and Optimising Clinical Trial Performance
23 January 2024 | Copthorne Tara Hotel, London, UK
Leveraging technology within clinical trials has been proven to reduce errors by 50% whilst improving efficiency, accuracy, and cost-effectiveness.
However, a slower-than-anticipated adoption of technology in clinical trials can be attributed to several factors, including an uncertain ROI, internal resistance to change, and insufficient consideration of patient experience in Digital Health Tool design.
Discover how you can embrace cutting-edge technology to improve patient retention, leverage data insights, and enhance clinical trial performance by joining our clinical trials technology leaders from the top pharmaceuticals and CROs across Europe on 23 January 2024.
Expert Clinical Trial Technology Speakers Include:

Dr. Sabrina Grant
Principal Sensor Solutions Leader
Parexel

Dr Sachin S Shah
Digital Biomarker Lead
GSK
Daragh Ryan
Former Senior Director Janssen Clinical Innovation
The Janssen Pharmaceutical Companies of Johnson & Johnson

Zoe Ashmore
Sensor Solutions Director
Parexel
Dr Fabian Kreimendahl
Medical Evidence Biostatistics Manager
The Janssen Pharmaceutical Companies of Johnson & Johnson
Key Themes Include:
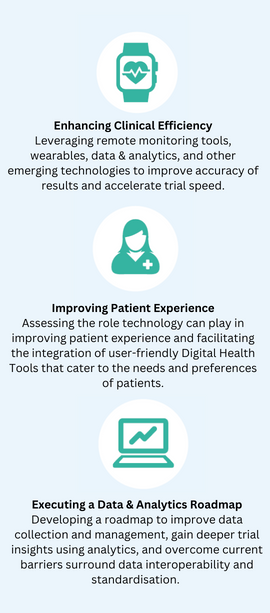
Attending this event will enable you to:
- Uncover the potential of emerging medical technologies and their capacity to revolutionise innovation in clinical trials
- Receive expert guidance on effectively incorporating technology such as remote monitoring tools, wearables and Artificial Intelligence into your clinical trials
- Improve real-world data collection techniques and optimise your overall data strategy
- Enhance your patient’s experience through increasing digital patient engagement and selecting patient centric medical devices
- Synchronise and synergise internal stakeholders through instilling an organisational culture of innovation within your Clinical Trials
Who You Will Meet:
VPs, Directors, Heads, Managers from:
- Clinical R&D / Operations
- Clinical Trial Innovation
- Clinical Development
- Digital Health / Digital Medicine
- DCT / Decentralised clinical trials / hybrid trials
- Patient Experience, Patient Engagement / Patient-centred Outcomes
- Regulatory / Clinical Affairs
- Biostatistics / biomarkers / bioinformatics / data generation / data strategy
Our sponsors include technology, service and solution providers specialising in:
- Remote-monitoring tools
- Wearables / Biosensors
- Data Analytics Software & Platforms
- Patient Experience Management Platforms / Mobile & Web Applications
- AI (Artificial Intelligence) / ML (Machine Learning
- ePRO / Electronic patient-reported outcome
- eCOA / Electronic Clinical Outcome Assessment
- eSource / Electronic Data Capture / DDC Direct Data Capture
- eConsent / Digital Enrolment